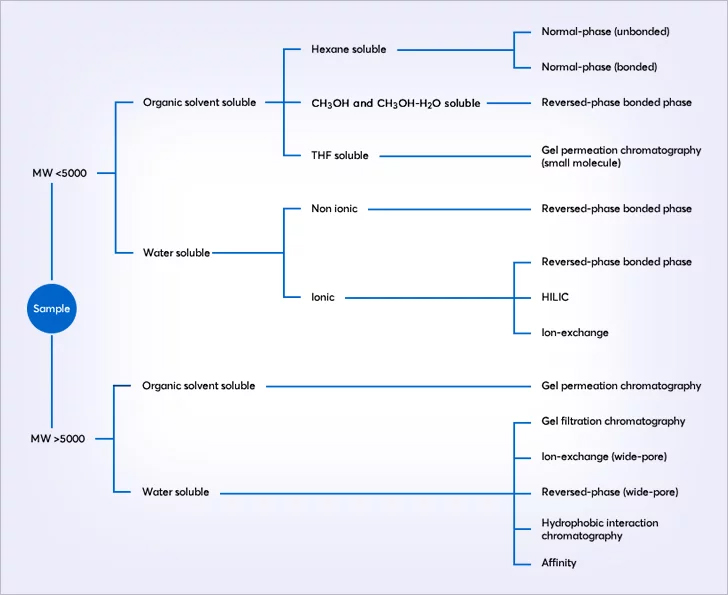
Liquid column chromatography selection overview
Base Material
- Silica is the most popular base material. It has a high physical strength and a surface which is easily chemically modified to give phases suitable for use in a broad range of HPLC modes. However, silica dissolves in water at pH ≥6.5, whilst bonded silicas are prone to be unstable at pH ≤2.5. Newer bonded silicas may have an extended pH range of 2 -10 or higher. More recent innovations include the TYPE-C™ Silica range in which the silica surface is modified with a layer of silicon hydride
- Polymeric materials have minimal pH restrictions but are less physically stable and exhibit lower separation efficiencies than silica for small molecules. For large molecules such as proteins or synthetic polymers, their performance is comparable to that of silica based materials.
- Graphitised carbon has high strength and excellent pH stability but cannot be modified. It can be expensive and is best used in unique selectivity applications.
- Zirconia (ZrO2) has the advantage of unique selectivity combined with extreme chemical and thermal stability (up to 200°C)
- Titania (TiO2) is stable over a wide pH range and at elevated temperatures. In contrast to silica, the surface of titania is alkaline, which can be beneficial in the analysis of basic drugs, but separation efficiencies are generally lower
- Alumina has greater pH stability than silica but cannot be easily chemically modified
Separation Mode
In simple terms, selection of the appropriate chromatographic separation mode is guided by the solute’s molecular size and polarity. An outline selection guide is given below. For some molecules, more than one technique may be appropriate.